PCR is a very sensitive technique that allows amplification of target sequences from very small amounts of input DNA. However, this sensitivity also makes PCR prone to contamination. Contamination may lead to incorrect data interpretation and false positive results. This summary explains the causes of PCR contamination and presents effective strategies to prevent it.
What is PCR contamination?
PCR contamination occurs when unwanted DNA sequences are introduced into a PCR reaction. This can happen at various steps of the DNA testing process, but most often it is caused by:
- Cross-contamination: Physical contact between samples, reagents, and equipment can transfer DNA between sources.
- Carry-over contamination: tiny droplets carrying PCR products from previously completed reactions can act as templates in subsequent reactions.
Always run a negative control
Ensuring valid diagnostic results requires detecting PCR contamination. A key tool is the negative control, which contains all PCR components except the template DNA. This serves as a baseline reference and should show no amplification. If a PCR product appears (fluorescence in fluoPCR or bands in gelPCR), this means that contamination is present and must be addressed.
What are the consequences of PCR contamination?
The consequences of PCR contamination can be severe and may include:
- False positive results: Contaminating DNA can lead to the incorrect identification of the target sequence or the detection of nonexistent targets.
- Reduced sensitivity: Contamination may dilute the target DNA, resulting in lower sensitivity and failure to detect low-abundance targets.
Minimizing PCR contamination: best practices
An organized lab workflow is key to reducing the risk of PCR contamination.
Crucial points include:
- To minimize contamination, maintain distinct workspaces for each step of the DNA test::
- Reagents area: for handling and preparing PCR reagents only.
- Sampling area: for sample preparation and DNA extraction.
- PCR area: for amplification and result visualization (e.g. DNA gel electrophoresis or fluorescence detection)
- Maintain a linear workflow from the reagents area, sampling area, to the PCR and viewing area, minimizing exchange between areas.
- Dedicate specific micropipettes, racks, tip boxes, trash bins, gloves, and lab coats to each area.
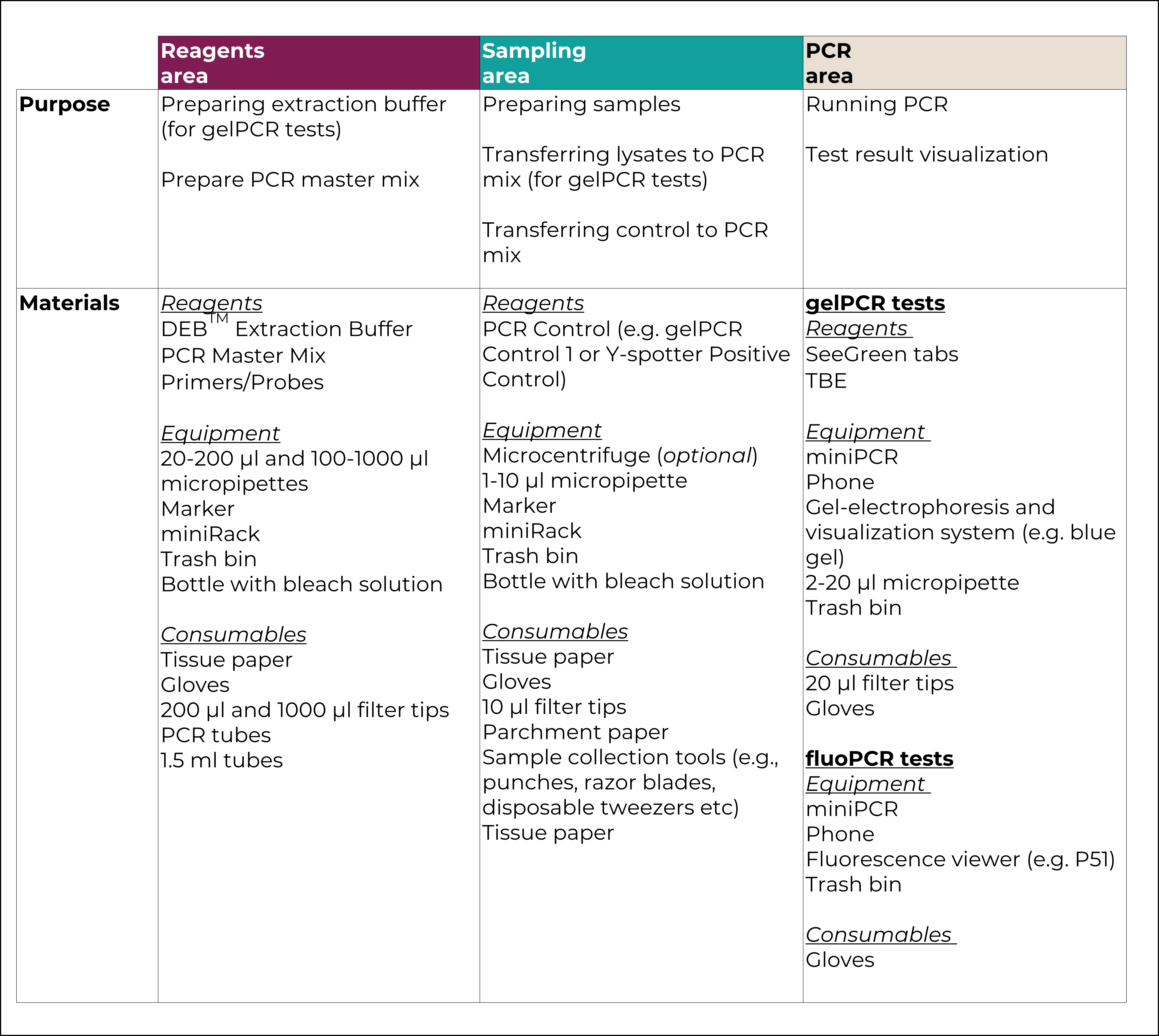
The table explains how to organize your lab to prevent PCR contamination.
Implement these strategies
- Wipe surfaces with freshly prepared 5% bleach before and after working in a certain area.
- When handling PCR tubes, make sure that the liquid is at the bottom of the tubes before opening them.
- Open only one tube at the time and make sure to seal them properly.
- Use of filter tips: filter tips act as barriers, preventing aerosols from entering pipettes and reducing the risk of contamination.
- Regularly discard trash that has been in contact with DNA sources (e.g., collect used tips and punches used during a test into a designated container and empty it often).
- Use of disposable punches, tweezers, or blades to collect samples.
- Avoid starting an experiment if your clothes are potentially contaminated with target DNA sources (e.g. coming from the husbandry or the greenhouse).
- Aliquoting reagents: divide and aliquot reagents into “mono-use amount” to reduce the likelihood of contaminating entire stocks.
How can I manage PCR contamination incidents?
In the event of contamination, immediate actions can mitigate its impact.
- Discard ALL contaminated reagents and consumables: dispose of any reagents suspected of contamination, including master mixes, primers mixes, and buffers.
- Clean lab coats and workspace: launder lab coats and disinfect work surfaces with 10% bleach to eliminate potential contaminants.
- Use new consumables: discard the opened tips boxes and tube bags and switch to fresh, uncontaminated tips to prevent any carryover.
- Keep track of the contamination incidents to determine if a systematic mistake/practice is happening.
In conclusion, it is important to always monitor potential PCR contamination through the negative control reaction. Understanding the potential sources for PCR contamination and implementing a strict workflow mitigates the contamination risk.